Chapter 5 - Wave Theory and
the Electron
(Continued —
Page 4)
Printer-Friendly Version
Molecule Bonds
Single energy formations like atoms must join others to create
a closed swirl formation that is capable of stabilizing energy.
In this formation, in addition to the each atom’s individual
swirl, there is a common central swirl that maintains energy
in a stronger fashion than the single atoms did. These central
swirls can be highly energetic, as in atoms, or weakly magnetic,
as in molecules. Every closed formation has one purpose: to
maintain energy, which is inflationary and tries to escape
into space. The following picture shows the molecule connections:
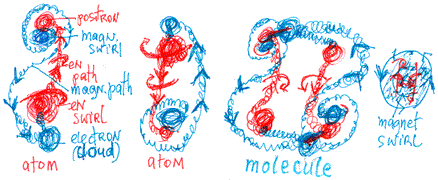
From this picture, we see that adding energy elongates the
magnetic path and enlarge the space between the electron and
the energetic swirl. The electron cloud comes in contact with
the positron cloud in front of the magnetic swirl of a neighbouring
atom, creating a large, high-energy positron-electron wave-like
formation. By adding energy, the electron can be easily separated
from the positron of the second atom; the electron will not,
however, escape from its own atom. An enormous amount of energy
is required to enlarge the electron’s space and enable
it to escape the atom’s wave.
Every atom must gather its characteristic requirement of
energy to jump to a new orbit. This orbit is a new, fluctuating
energetic path, connected by double paths to the magnetic
and energetic swirls.
Back to
Top
Dr. Chaim Tejman, Copyright©
2001. All rights reserved.
[Index]
[Introduction]
[Summary] [Wave
Formation] [Photons] [Gravitation]
[Time]
[Atoms] [Life]
[Cancer] [Fundamental
Force] [Gender/Why Sex?]
[Sexual Reproduction]
[Schrodinger & Heisenberg]
[Creation] [Supernova]
[Dark
Matter & Astronomy] [Speed
of Light] [Cloud Formations]
[Natural Disasters] [Global
Warming] [Thermodynamics]
[Backward Time] [Quantum
Mechanics] [Compton Effect]
[Equations] [Predictions]
[Academic Correspondences] [Contact]
[Links] [Mysteries] |